After the Precision Medicine Strategy was introduced and implemented, many research universities and medical institutions have been building their own biobanks. However, there are significant differences of sample management and sample quality among biobanks because of lack of communication and professional biobank designs.

In the reproductive center of a hospital in China, the original data was covered by other data, and doctors were not sure who the real owners of examination reports were. At last, they took samples from patients and did examination again for patients.
Another example is from a cord blood biobank. When an operator changed the sample time without permission, all information about this sample was lost. For the cord blood owner, the hope of treating future diseases was broken.
The website Mediapart published fragments from a leaked letter written by two French Cabinet ministers who listed a series of apparent problems of Institute Pasteur. "High likelihood of [sample] destruction not ordered by managers and without traceability,

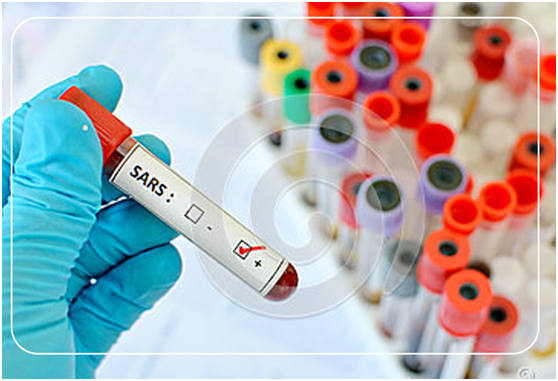
Because of problems of sample management, Institute Pasteur could not account for 2349 vials containing samples from the SARS outbreak in 2003. This caused a panic in the whole world. Biologix Corporation, the professional of biobanking services expressed that a strict procedure was needed to manage samples, and a complete biobank Standard Operating Procedure was crucial.
CryoKING Complete Biobanking Solutions
CryoKING is a cold chain and biobanking brand by Biologix Corporation. CryoKING products focus and concentrated on standardization,